Varied Adsorbents For The Chromatographic Separation Process
Chromatography is a powerful and well known technique that is widely used to separate and purify various mixtures of substances based on their different physical or chemical properties. The process can provide high quality results for the separation of compounds from a mixture due to its high adsorption capabilities, since it has a very porous structure, that can adsorb different compounds according to their polarity and affinity properties towards the desiccant material used in the process.
Adsorption is the process of selectively attracting and retaining molecules from a sample mixture, and plays a crucial role in the chromatography process. Different types of adsorbents with different properties are used to separate mixtures based on factors like polarity, size and charge. Thus different adsorbents for the chromatographic separation will provide different results due to their unique characteristics and properties to separate the compounds and the particular chromatography technique used.
The selection of the appropriate adsorbent is critical for achieving effective separation results in chromatography. You need to consider various factors such as the polarity of the compounds to be separated, the desired separation efficiency, the sample characteristics, and cost and availability when choosing the particular separation process.
Types of adsorbents used in a chromatographic separation process
Different adsorbent materials have unique properties such as varying pore size, affinity, absorbency levels, and polarity. Thus it is essential to choose the right adsorbent material for the appropriate chromatographic separation process.
Silica gel
This is one of the most commonly used adsorbent materials in the chromatographic separation process. This is because it is easily available, has a highly porous structure, and can effectively separate the compounds from any mixture, and also void them of any contaminants and impurities.
Its high surface area and variable pore sizes make it suitable for a broad range of applications in chromatography. Silica gel, which is made of hydrated silicon dioxide, is particularly effective in normal-phase chromatography, where the separation is based on differences in polarity.
The surface of silica gel is hydrophilic in nature and can attract polar compounds. Silica gel is available in various grades with different pore sizes and activity levels. The pore size you choose for the chromatography process, determines the size of molecules that can be adsorbed, while the activity level affects the strength of adsorption.
Alumina/ Activated Alumina
The Alumina material is composed of aluminium oxide, which is another common adsorbent employed in many chromatography techniques. Like silica gel, activated alumina is versatile and finds application in both normal-phase and reversed-phase chromatography processes. Since alumina is an amphoteric adsorbent, meaning it can act as both an acid and a base, and its distinct properties make it especially useful for separating compounds with similar polarities.
It is a highly porous material made of aluminum oxide, and the surface of Al2O3 can be modified to make it acidic, neutral, or basic and be applied to various chromatography techniques and processes effectively. The type of alumina used depends on the polarity of the compounds to be separated.
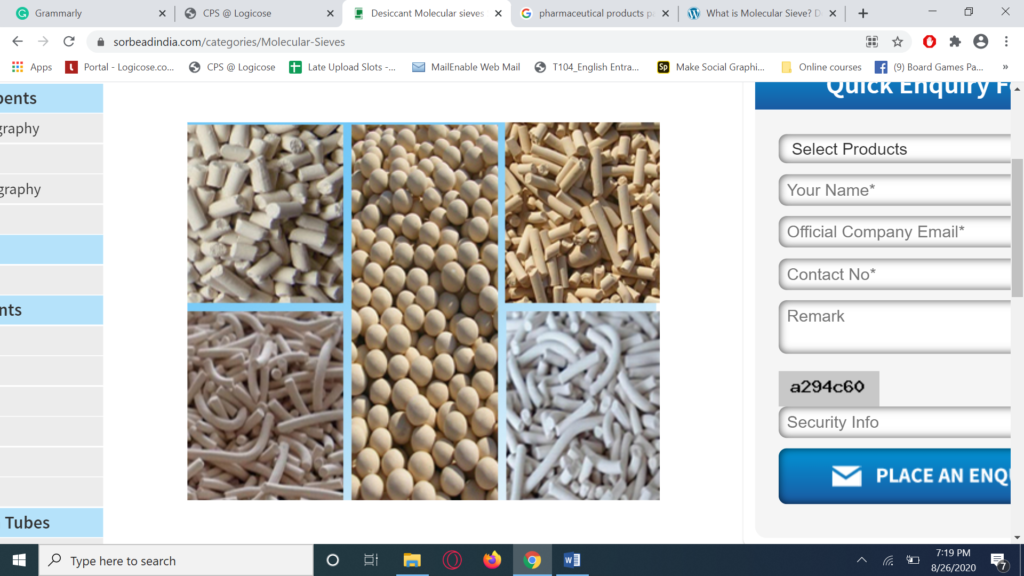
Molecular sieves
Molecular sieves are generally crystalline aluminosilicate materials, with high surface area, that have a well defined porous structure, and are widely used in many chromatographic techniques to separate compounds in a mixture. The Zeolite structure is often used in gas chromatography to selectively adsorb particular gases based on their size and polarity, making them essential for the separation of complex gas mixtures at high levels of accuracy.
Molecular sieves are also used to dry solvents and eluents in chromatography to prevent unwanted interactions between the solvent and the stationary phase or the sample components, and in reversed-phase chromatography, for the separation of nonpolar compounds.
The polarity of the molecular sieve can be modified to achieve the desired selectivity according to the separation of compounds you want to achieve. Zeolites also have high thermal stability and are also resistant to most chemicals, making them suitable for use with various solvents and eluents.
Activated charcoal
Activated charcoal is a type of nonpolar adsorbent that is very often used for non-aqueous reversed-phase chromatography. It is a porous material made of carbon that has been activated by heating it in a controlled atmosphere. It is derived from carbon-rich materials like wood, peat, coconut shells, or sawdust, and works as a potent and distinctive adsorbent in many other chromatography techniques such as liquid and gas chromatography.
The activation process creates a large surface area with many pores, making activated charcoal highly adsorptive. Activated charcoal is effective in removing impurities and contaminants from various solutions, including dyes, pesticides, and pharmaceuticals.
Factors affecting adsorbent selection for the separation process using chromatography
– The polarity of the adsorbent material should match or complement the polarity of the compounds to be separated using the chromatography process. For normal-phase chromatography, polar adsorbents like silica gel and alumina are suitable for separating polar compounds, while nonpolar adsorbents like activated charcoal are better suited for nonpolar compounds. In reversed-phase chromatography, the polarity relationship is reversed.
– The adsorbent must have the right pore size and level of activity to achieve the desired adsorption efficiency. A smaller pore size is better for smaller molecules, while a larger pore size is more effective for larger molecules. Activity levels determine adsorption efficiency, with higher activity levels resulting in better retention of compounds.
– The solubility and volatility of analytes that are used in the particular chromatography process play an important role in the selection of a suitable adsorbent for a particular type of chromatography. For example, in gas chromatography, where volatile compounds are separated based on their vapor pressures, the choice of an adsorbent material with high thermal stability becomes an essential factor to be considered.
– The size of the molecules involved in the separation is a critical factor. Larger molecules may be better suited for size exclusion chromatography, where highly porous adsorbents allow smaller molecules to enter the pores, resulting in separation based on size. But, smaller molecules may be efficiently separated using adsorbents with smaller pores.
– The mobile phase’s polarity affects how the mobile phase interacts with the adsorbent material that is used and the analytes. When a polar mobile phase is used in reverse-phase chromatography the polarity of the adsorbent must be complementary to the mobile phase for effective separation.
Selecting the right adsorbents for the chromatographic separation using specific chromatography technique is essential. By taking into account these considerations and factors, you can select the best adsorbent for their particular separation needs.